Question 8:
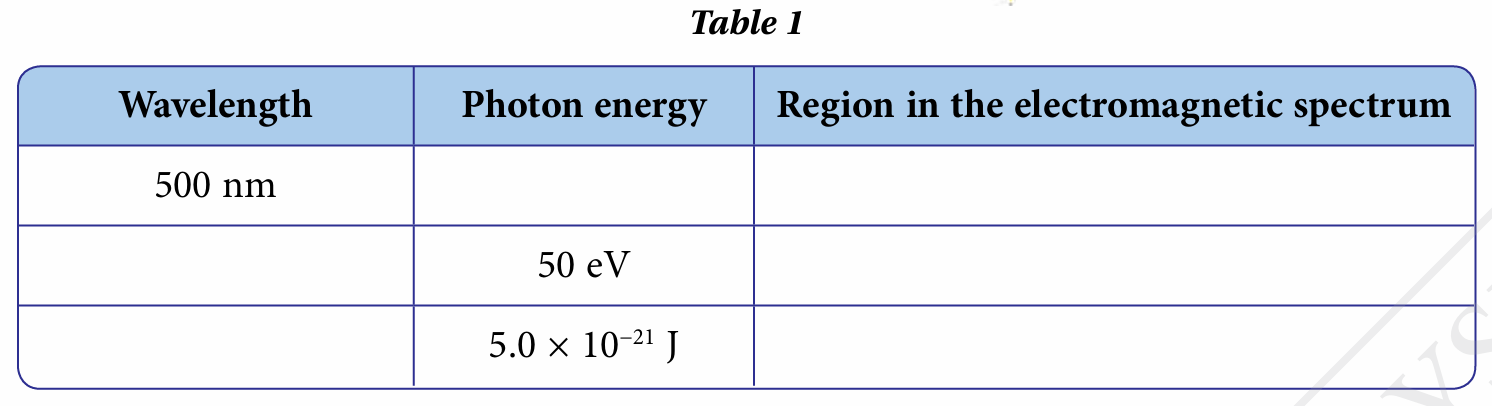
Complete Table 1 with information on the wavelength and photon energy for several components of waves in the electromagnetic spectrum.
Answer:
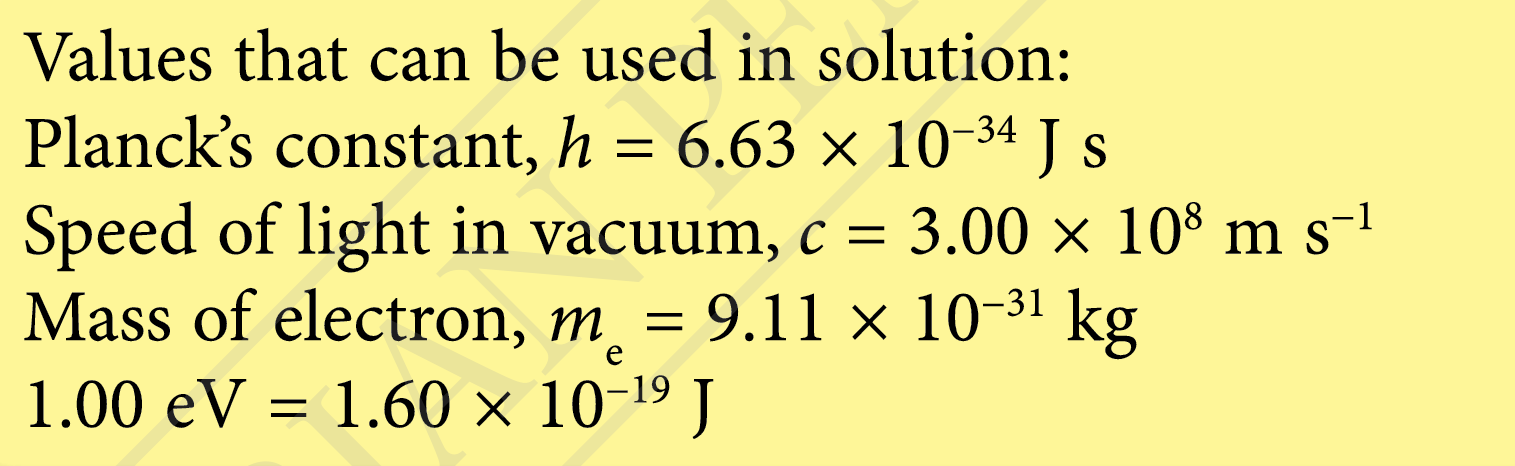
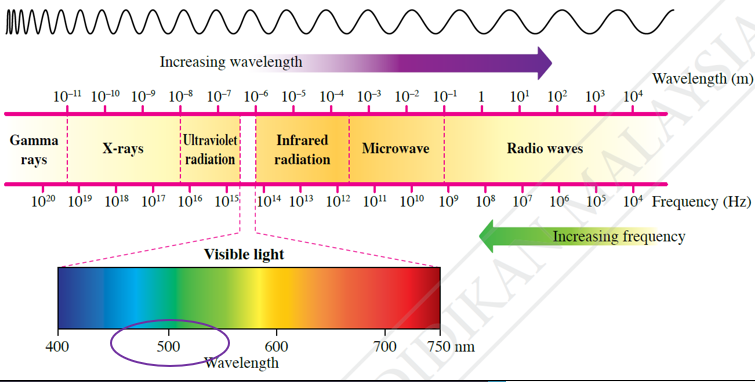
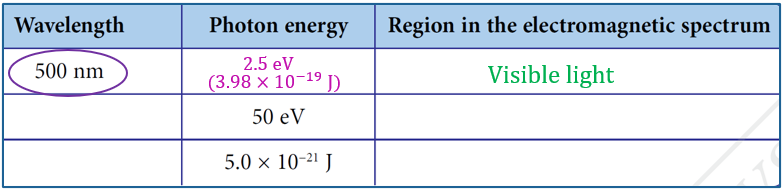
$$ \begin{aligned} E & =\frac{h c}{\lambda} \\ & =\frac{\left(6.63 \times 10^{-34}\right)\left(3.00 \times 10^8\right)}{500 \times 10^{-9}} \\ & =3.98 \times 10^{-19} \mathrm{~J} \\ & =\frac{3.98 \times 10^{-19} \mathrm{~J}}{1.60 \times 10^{-19} \mathrm{~J}} \\ & =2.5 \mathrm{eV} \end{aligned} $$
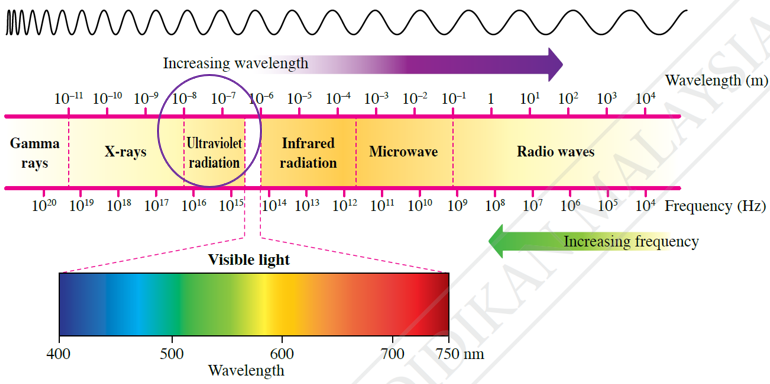
$$ \begin{aligned} \lambda_1 & =\frac{h c}{E} \\ & =\frac{\left(6.63 \times 10^{-34}\right)\left(3.00 \times 10^8\right)}{50 \times 1.6 \times 10^{-19}} \\ & =2.49 \times 10^{-8} \mathrm{~m} \\ & =25 \mathrm{~nm} \end{aligned} $$
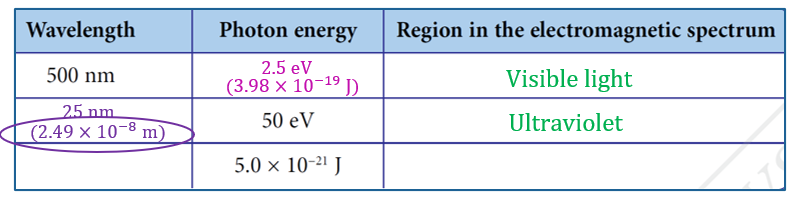
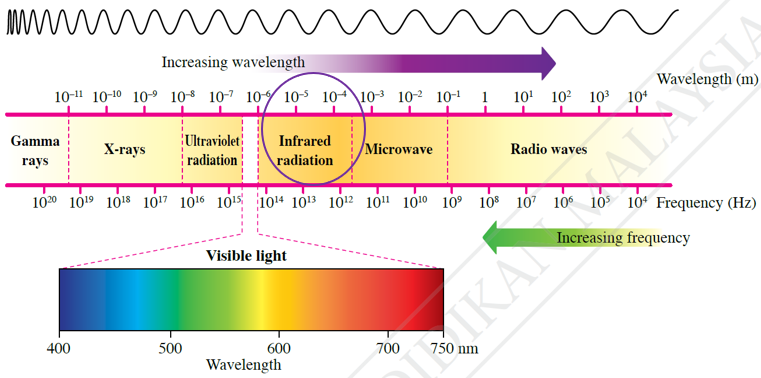
$$ \begin{aligned} \lambda_2 & =\frac{h c}{E} \\ & =\frac{\left(6.63 \times 10^{-34}\right)\left(3.00 \times 10^8\right)}{5.0 \times 10^{-21}} \\ & =3.98 \times 10^{-5} \mathrm{~m} \\ & =40 \mu \mathrm{m} \end{aligned} $$
Complete Table 1 with information on the wavelength and photon energy for several components of waves in the electromagnetic spectrum.

Answer:
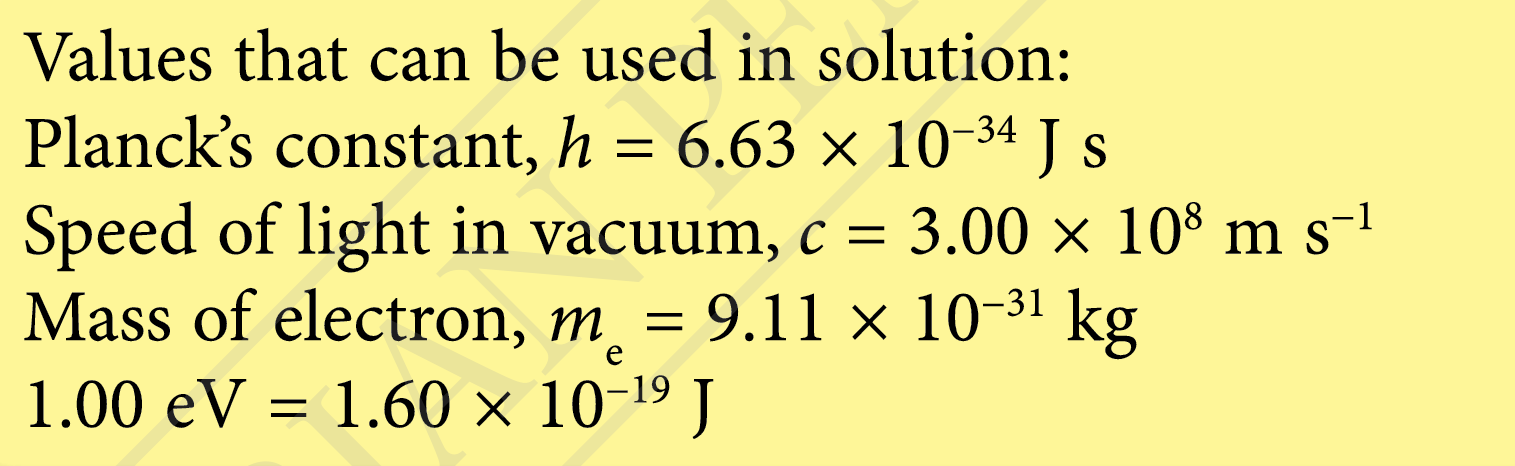

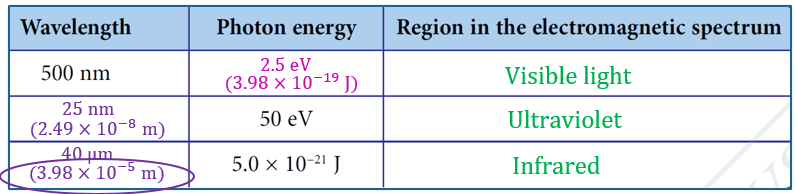
$$ \begin{aligned} E & =\frac{h c}{\lambda} \\ & =\frac{\left(6.63 \times 10^{-34}\right)\left(3.00 \times 10^8\right)}{500 \times 10^{-9}} \\ & =3.98 \times 10^{-19} \mathrm{~J} \\ & =\frac{3.98 \times 10^{-19} \mathrm{~J}}{1.60 \times 10^{-19} \mathrm{~J}} \\ & =2.5 \mathrm{eV} \end{aligned} $$

$$ \begin{aligned} \lambda_1 & =\frac{h c}{E} \\ & =\frac{\left(6.63 \times 10^{-34}\right)\left(3.00 \times 10^8\right)}{50 \times 1.6 \times 10^{-19}} \\ & =2.49 \times 10^{-8} \mathrm{~m} \\ & =25 \mathrm{~nm} \end{aligned} $$


$$ \begin{aligned} \lambda_2 & =\frac{h c}{E} \\ & =\frac{\left(6.63 \times 10^{-34}\right)\left(3.00 \times 10^8\right)}{5.0 \times 10^{-21}} \\ & =3.98 \times 10^{-5} \mathrm{~m} \\ & =40 \mu \mathrm{m} \end{aligned} $$


