Quiz Summary
0 of 11 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 11 questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- Answered
- Review
-
Question 1 of 11
1. Question
1 point(s)Which of the followings are the advantageous of aneroid barometers over simple barometers in their use to measure atmospheric pressure?
CorrectIncorrect -
Question 2 of 11
2. Question
1 point(s)Which of the following instrument can be used to measure atmospheric pressure?
CorrectIncorrect -
Question 3 of 11
3. Question
1 point(s)A gas in a container exerts a pressure on the wall of the container because the molecules of the gas ________.
CorrectIncorrect -
Question 4 of 11
4. Question
1 point(s)Which of the followings are the assumptions of kinetic theory of gas?
CorrectIncorrect -
Question 5 of 11
5. Question
1 point(s)Which of the followings do not apply the concept of atmospheric pressure in their working mechanism?
CorrectIncorrect -
Question 6 of 11
6. Question
1 point(s)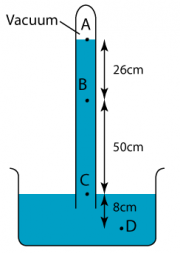
The diagram shows a simple barometer filled with mercury. What is the pressure at point D?
CorrectIncorrect -
Question 7 of 11
7. Question
1 point(s)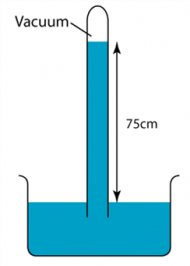
The diagram shows a simple barometer filled with mercury placed in a classroom. Find the atmospheric pressure of the classroom. (Density of mercury = 13,600 kg/m3)
CorrectIncorrect -
Question 8 of 11
8. Question
1 point(s)
The figure above shows 2 identical tubes with one end immersed in mercury. Tube Y is located higher than tube X, and the mercury column of tube Y in the image doesn’t show the actual level. What will be the level of the mercury column of tube Y if the space inside is vacuum?
CorrectIncorrect -
Question 9 of 11
9. Question
1 point(s)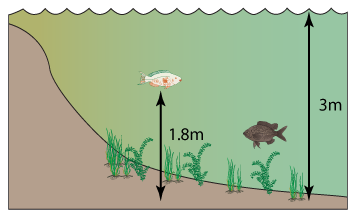
The diagram shows the cross-section of a lake. Find the pressure at the bottom of the lake in the unit of Pascal. (Density of water = 1000kg/m3; Atmospheric pressure = 100,000Pa.)
CorrectIncorrect -
Question 10 of 11
10. Question
1 point(s)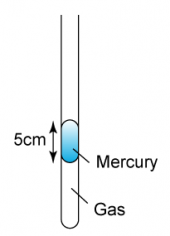
In Figure above, if the atmospheric pressure is 75 cmHg, what is the pressure of the trapped gas?
CorrectIncorrect -
Question 11 of 11
11. Question
1 point(s)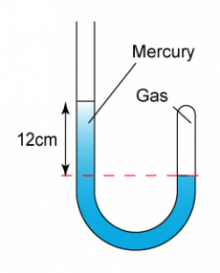
The diagram shows a J-Tube with some gas trapped in the mercury. Find the pressure of the gas trapped in the mercury. (Atmospheric pressure = 76 cmHg)
CorrectIncorrect
