Quiz Summary
0 of 11 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 11 questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- Answered
- Review
-
Question 1 of 11
1. Question
1 point(s)A glass of hot water is covered with an airtight lid and left to cool. Why is it difficult to lift the lid when the water is cold.
CorrectIncorrect -
Question 2 of 11
2. Question
1 point(s)The air pressure in a tyre is 240 kPa at a temperature of 20°C. Find the pressure of the air when the temperature increases to 50°C. (Give your answer correct to 3 significant figure)
CorrectIncorrect -
Question 3 of 11
3. Question
1 point(s)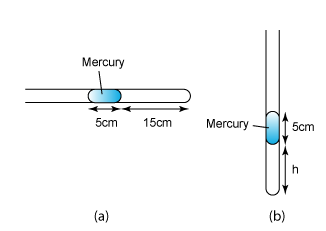
Figure (a) above shows some air is trapped in a capillary tube by a mercury column of 5cm. Figure (b) shows the condition when the capillary tube is held horizontal. Find the length, h, of the air trapped in (b). (Atmospheric pressure = 75cmHg)CorrectIncorrect -
Question 4 of 11
4. Question
1 point(s)A fish releases a bubble of air of volume 1cm3 at the bottom of a lake. The depth of the lake is 10m. Find the volume of the bubble when it reaches the surface of the pond. (Assume that the atmospheric pressure is equal to 10m of water)
CorrectIncorrect -
Question 5 of 11
5. Question
1 point(s)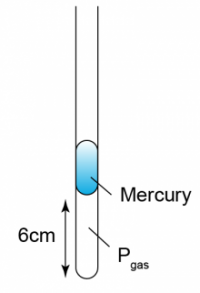
The figure shows some air trapped in a capillary tube. Given that the temperature of the air is 27°C. Find the length of the air column when the temperature of the air is increased to 87°C.CorrectIncorrect -
Question 6 of 11
6. Question
1 point(s)Which of the following graphs shows the relationship between pressure, P, and volume, V of a gas at constant temperature?
CorrectIncorrect -
Question 7 of 11
7. Question
1 point(s)Water take a shorter time to boil at a region of high altitude (Such as Genting Highland) compare with a region of low altitude although the same amount of energy is used. This is because
CorrectIncorrect -
Question 8 of 11
8. Question
1 point(s)Given that the boiling point of pure water is 100°C at room temperature and pressure. What will happen to the boiling point when some salt is added to the water?
CorrectIncorrect -
Question 9 of 11
9. Question
1 point(s)How much heat must be removed by a refrigerator from 500g of water at 25 °C to totally convert it to ice cubes at 0°C? [Specific heat capacity of water = 4200J kg-1 °C-1; Specific latent heat of fusion of ice = 334,000 Jkg-1]
CorrectIncorrect -
Question 10 of 11
10. Question
1 point(s)How much heat energy is needed to completely convert water of mass 1kg at 50°C to steam at 100°C? (Specific heat capacity of water = 4200 Jkg-1°C-1; Specific latent heat of vaporisation of water = 2,260,000 Jkg-1)
CorrectIncorrect -
Question 11 of 11
11. Question
1 point(s)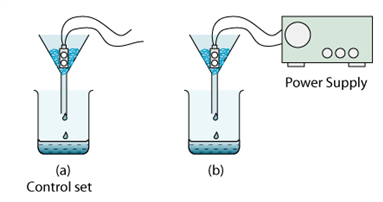
The figure above shows the experiment set up to determine the specific latent heat of fusion. What is the purpose of the control set in the experiment?CorrectIncorrect
