Question 4:
Pressure in a gas cylinder is 175 kPa at a temperature of 27°C. Heat from a nearby furnace causes the gas pressure to increase to 300 kPa. What is the temperature of the gas inside the cylinder?
Answer:
\begin{aligned} & P_1=175 \mathrm{kPa} \\ & P_2=300 \mathrm{kPa} \\ & T_1=27+273=300 \mathrm{~K} \\ & T_2=? \end{aligned}
Pressure in a gas cylinder is 175 kPa at a temperature of 27°C. Heat from a nearby furnace causes the gas pressure to increase to 300 kPa. What is the temperature of the gas inside the cylinder?
Answer:
\begin{aligned} & P_1=175 \mathrm{kPa} \\ & P_2=300 \mathrm{kPa} \\ & T_1=27+273=300 \mathrm{~K} \\ & T_2=? \end{aligned}
Gay-Lussac’s Law formula: $$ \begin{aligned} \frac{P_1}{T_1} & =\frac{P_2}{T_2} \\ \frac{175}{300} & =\frac{300}{T_2} \\ T_2 & =\frac{300 \times 300}{175} \\ & =514.29 \mathrm{~K}-273 \\ & =241.29^{\circ} \mathrm{C} \end{aligned} $$
Question 5:
Figure 4.35 shows an apparatus set up to study the relationship between pressure and temperature for air inside a round-bottom flask.
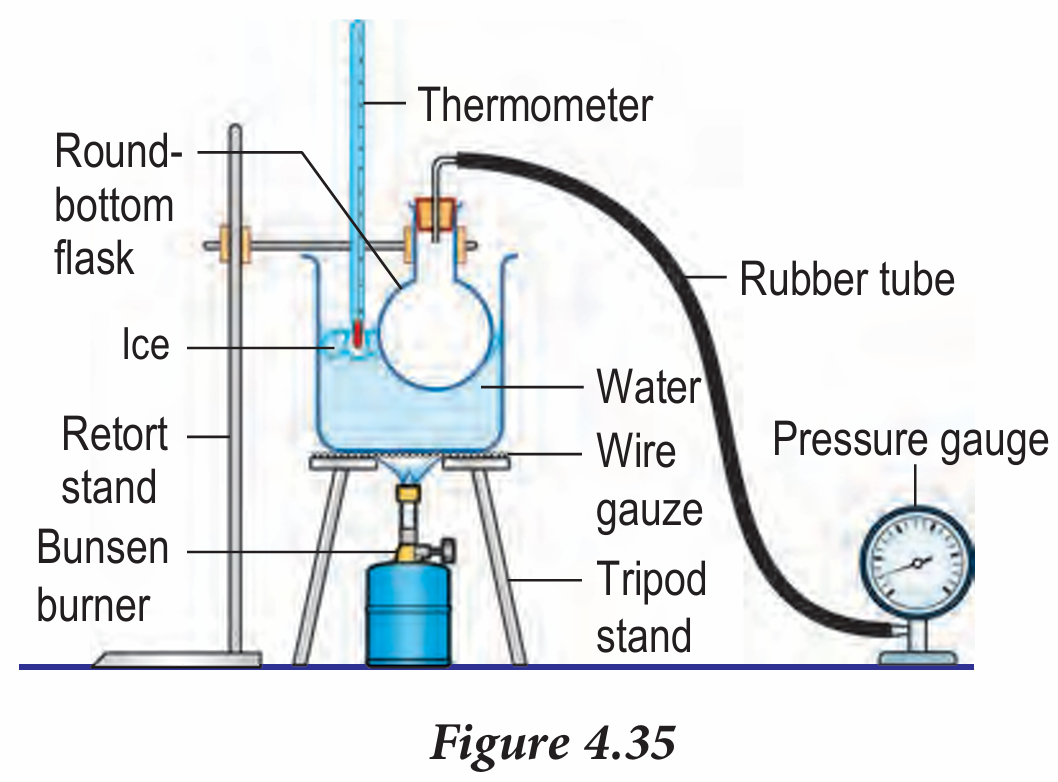
(a) Identify four aspects in the apparatus set up that can jeopardise the accuracy of the results of the experiment.
(b) Suggest modifications to improve the set up.
Answer:
(a)
(b)
Figure 4.35 shows an apparatus set up to study the relationship between pressure and temperature for air inside a round-bottom flask.

(a) Identify four aspects in the apparatus set up that can jeopardise the accuracy of the results of the experiment.
(b) Suggest modifications to improve the set up.
Answer:
(a)
- The round bottom flask is not fully immersed inside the water
- Position of thermometer is on the surface of water
- No stirrer rod
- Location of pressure gauge at lower level
(b)
- The round bottom flask must immerse deeper inside the water to ensure all the molecules of gas heat up uniformly.
- Immerse the mercury bulb of the thermometer inside the water to get more accurate reading.
- A stirrer rod is used to stir the water for spreading heat uniformly.
- The pressure gauge is put on the block to make it closer to the flask to prevent loss of gas pressure.
