Question 1:
What is the difference between heat capacity and specific heat capacity?
Answer:
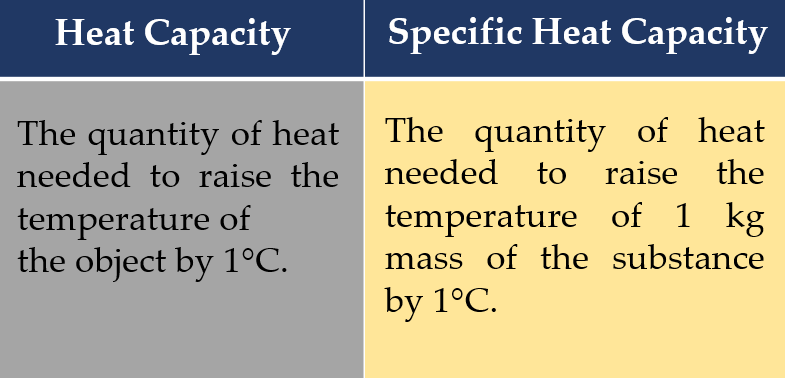
What is the difference between heat capacity and specific heat capacity?
Answer:

Question 2:
How much heat energy is needed to increase the temperature of a 0.2 kg mass of gold by 10°C?
[Given the value of specific heat capacity of gold is 300 J kg–1 °C–1]
Answer:
\begin{aligned} Q & =m c \theta \\ & =(0.2)(300)(10) \\ & =600 \mathrm{~J} \end{aligned}
How much heat energy is needed to increase the temperature of a 0.2 kg mass of gold by 10°C?
[Given the value of specific heat capacity of gold is 300 J kg–1 °C–1]
Answer:
\begin{aligned} Q & =m c \theta \\ & =(0.2)(300)(10) \\ & =600 \mathrm{~J} \end{aligned}
Question 3:
A container contains 200 g of water at initial temperature of 30°C. An iron nail of mass 200 g at temperature of 50°C is immersed in the water. What is the final water temperature? State the assumptions you need to make in your calculations.
[Given the value of specific heat capacity of water is 4 200 J kg–1 °C–1 and that of iron is 450 J kg–1 °C–1]
Answer:
Mass, mw = 200 g, θw = 30oC
Mass, mn = 200 g, cn = 4 200 J kg–1 °C–1
Specific heat capacity of water, cw = 4 200 J kg–1 °C–1
Specific heat capacity of iron, cn = 450 J kg–1 °C–1
Final water temperature, θf = ??? °C
$$ \begin{aligned} Q_w & =Q_n \\ m_w c_w \Delta \theta_w & =m_n c_n \Delta \theta_n \\ m_a c_a\left(\theta_{f}-\theta_w\right) & =m_n c_n\left(\theta_n-\theta_{f}\right) \\ (200)(4200)\left(\theta_{f}-30\right) & =(200)(450)\left(50-\theta_{f}\right) \end{aligned} $$
$$ \begin{aligned} \left(4200 \theta_{f}-126000\right) & =\left(22500-450 \theta_{f}\right) \\ 4200 \theta_{f}+450 \theta_{f} & =22500+126000 \\ 4650 \theta_{f} & =148500 \\ \theta_{f} & =31.94^{\circ} \mathrm{C} \end{aligned} $$
Assumption :
Heat released is the same as the heat absorbed.
A container contains 200 g of water at initial temperature of 30°C. An iron nail of mass 200 g at temperature of 50°C is immersed in the water. What is the final water temperature? State the assumptions you need to make in your calculations.
[Given the value of specific heat capacity of water is 4 200 J kg–1 °C–1 and that of iron is 450 J kg–1 °C–1]
Answer:
Mass, mw = 200 g, θw = 30oC
Mass, mn = 200 g, cn = 4 200 J kg–1 °C–1
Specific heat capacity of water, cw = 4 200 J kg–1 °C–1
Specific heat capacity of iron, cn = 450 J kg–1 °C–1
Final water temperature, θf = ??? °C
$$ \begin{aligned} Q_w & =Q_n \\ m_w c_w \Delta \theta_w & =m_n c_n \Delta \theta_n \\ m_a c_a\left(\theta_{f}-\theta_w\right) & =m_n c_n\left(\theta_n-\theta_{f}\right) \\ (200)(4200)\left(\theta_{f}-30\right) & =(200)(450)\left(50-\theta_{f}\right) \end{aligned} $$
$$ \begin{aligned} \left(4200 \theta_{f}-126000\right) & =\left(22500-450 \theta_{f}\right) \\ 4200 \theta_{f}+450 \theta_{f} & =22500+126000 \\ 4650 \theta_{f} & =148500 \\ \theta_{f} & =31.94^{\circ} \mathrm{C} \end{aligned} $$
Assumption :
Heat released is the same as the heat absorbed.
