Question 1:
State the physical quantities that are constant in Boyle’s Law, Charles’ Law and Gay-Lussac’s Law.
Answer:
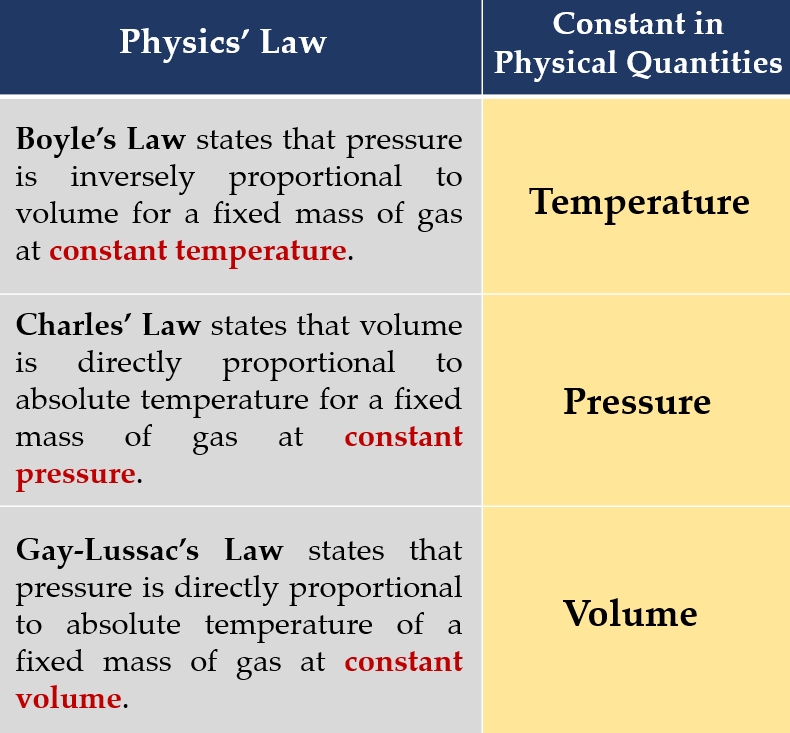
State the physical quantities that are constant in Boyle’s Law, Charles’ Law and Gay-Lussac’s Law.
Answer:

Question 2:
A syringe contains 50 cm3 of air at a pressure of 110 kPa. Th e end of the syringe is closed and its piston slowly pushed until the volume of air becomes 20 cm3. What is the pressure of the compressed air?
Answer:
\begin{aligned} & V_1=50 \mathrm{~cm}^3 \\ & V_2=20 \mathrm{~cm}^3 \\ & P_1=110 \mathrm{kPa} \\ & P_2=? \end{aligned}
\begin{aligned} P_1 V_1 & =P_2 V_2 \\ 110 \times 50 & =P_2(20) \\ P_2 & =\frac{110 \times 50}{20} \\ & =275 \mathrm{kPa} \end{aligned}
A syringe contains 50 cm3 of air at a pressure of 110 kPa. Th e end of the syringe is closed and its piston slowly pushed until the volume of air becomes 20 cm3. What is the pressure of the compressed air?
Answer:
\begin{aligned} & V_1=50 \mathrm{~cm}^3 \\ & V_2=20 \mathrm{~cm}^3 \\ & P_1=110 \mathrm{kPa} \\ & P_2=? \end{aligned}
\begin{aligned} P_1 V_1 & =P_2 V_2 \\ 110 \times 50 & =P_2(20) \\ P_2 & =\frac{110 \times 50}{20} \\ & =275 \mathrm{kPa} \end{aligned}
Question 3:
An air bubble trapped under a leaf in a lake has a volume of 1.60 cm3 at a temperature of 38°C. Calculate the volume of the bubble if the temperature of the water in the lake drops to 26°C.
Answer:
\begin{aligned} & T_1=38+273=311 \mathrm{~K} \\ & T_2=26+273=299 \mathrm{~K} \\ & V_1=1.60 \mathrm{~cm}^3 \\ & V_2=? \end{aligned}
An air bubble trapped under a leaf in a lake has a volume of 1.60 cm3 at a temperature of 38°C. Calculate the volume of the bubble if the temperature of the water in the lake drops to 26°C.
Answer:
\begin{aligned} & T_1=38+273=311 \mathrm{~K} \\ & T_2=26+273=299 \mathrm{~K} \\ & V_1=1.60 \mathrm{~cm}^3 \\ & V_2=? \end{aligned}
Charles’ Law formula: $$ \begin{aligned} \frac{V_1}{T_1} & =\frac{V_2}{T_2} \\ \frac{1.60}{311} & =\frac{V_2}{299} \\ V_2 & =\frac{1.60 \times 299}{311} \\ V_2 & =1.54 \mathrm{~cm}^3 \end{aligned} $$
