Quiz Summary
0 of 11 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 11 questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- Answered
- Review
-
Question 1 of 11
1. Question
1 point(s)2 solid blocks A and B of equal mass are heated by an electronic stove. The initial temperature of the 2 blocks is the same and heated by the same amount of energy. It is observed that the temperature of block A increased faster than block B. This observation is owing to the difference of the ______________ of the 2 blocks.
CorrectIncorrect -
Question 2 of 11
2. Question
1 point(s)Heat energy is supplied at the same rate to 500 g of olive oil and 500 g of water in similar containers. The temperature of the olive oil rises faster. This is because the olive oil
CorrectIncorrect -
Question 3 of 11
3. Question
1 point(s)Water is used as a cooling agent in the radiator of the vehicles because of its
CorrectIncorrect -
Question 4 of 11
4. Question
1 point(s)45 000 J of heat energy raises the temperature of a 2 kg block of a metal from 30 °C to 45°C. What is the specific heat capacity of the metal?
CorrectIncorrect -
Question 5 of 11
5. Question
1 point(s)Some lead shots of mass m are placed at the bottom of a vertical cylinder that is 1.2 m long and closed at both ends. The cylinder is suddenly inverted so that the shot falls 1.2 m. By how much will the temperature of the shot increase if this process is repeated 1000 times? Assume no heat loss. [The specific heat capacity of lead is 130Jkg⁻¹°C⁻¹]
CorrectIncorrect -
Question 6 of 11
6. Question
1 point(s)An electric heater supplies 2.5 kW of power in the form of heat to a pail of water. How long will it take to heat the 10 kg of water in the tank from 25 to 100 °C? Assume no heat losses to the surroundings. [Specific heat capacity of water = 4200 J kg⁻¹ °C⁻¹]
CorrectIncorrect -
Question 7 of 11
7. Question
1 point(s)2 iron blocks, P and Q are in thermal contact. The initial temperature of P and Q is 10°C and 50°C respectively. Which of the followings is true when P and Q are at thermal equilibrium.
CorrectIncorrect -
Question 8 of 11
8. Question
1 point(s)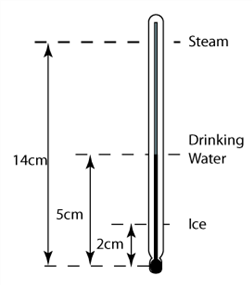
A student using an unmarked liquid-in-glass thermometer, puts the bulb into melting ice, then into steam above boiling water and finally into a drinking water. He marks the level of the liquid in the thermometer each time. The liquid levels, measured from the bulb, are shown on the diagram above. What is the temperature of the drinking water?
CorrectIncorrect -
Question 9 of 11
9. Question
1 point(s)The lengths of mercury thread in the uniform tube above the bulb of a mercury thermometer are:
30 mm when the bulb is in melting ice;
130 mm when the bulb is in the steam above boiling water;
70 mm when the bulb is in a liquid X.What is the temperature of liquid X?
CorrectIncorrect -
Question 10 of 11
10. Question
1 point(s)The sensitivity of a liquid-in-glass thermometer can be increased by
CorrectIncorrect -
Question 11 of 11
11. Question
1 point(s)Which of the following liquid is suitable to be used in a liquid-in-glass thermometer designed to measure temperatures from -50°C to 120°C.
CorrectIncorrect
