Question 4:
During the formation of rocks, radioisotope uranium-238 is trapped. The decay rate of uranium-238 is low and the end result of the decay series is lead-206. Table 1 shows the composition of samples of rock A and rock B.
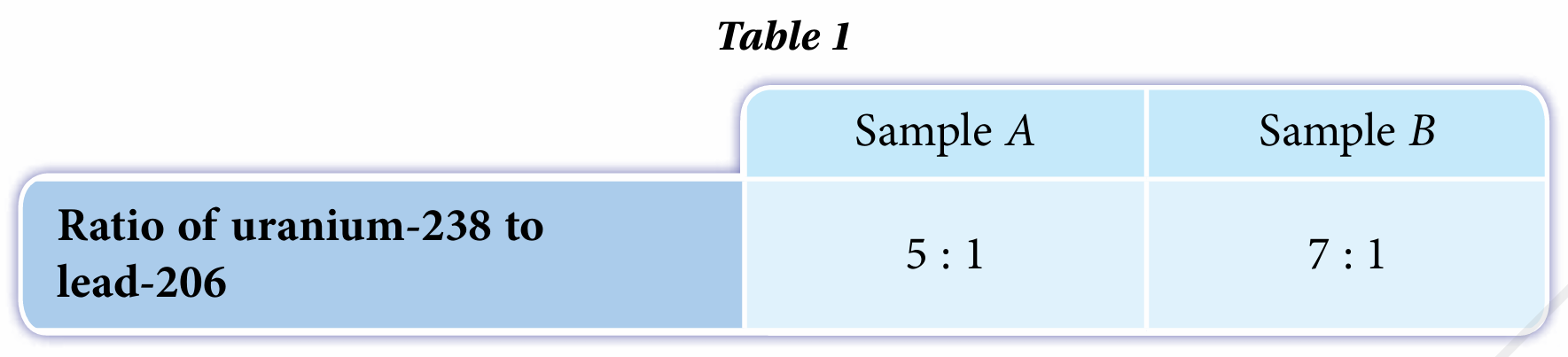
(a) Between the samples of rock A and rock B, which one is older? Justify your answer.
(b) The composition of the lead nucleus is unlikely to be greater than the uranium nucleus in the rocks sample. Explain your answer.
Answer:
(a)

The half-life of uranium-238 is 4.5 billion years. Therefore, the decay process of uranium -238 in a rock sample has gone through less than one half-life.
Hence, less than half of the uranium-238 nuclei in the sample of rock had decayed to form lead-206 nuclei.
So, the number of lead-206 nuclei cannot be more than the remaining uranium- 238 nuclei.
During the formation of rocks, radioisotope uranium-238 is trapped. The decay rate of uranium-238 is low and the end result of the decay series is lead-206. Table 1 shows the composition of samples of rock A and rock B.

(a) Between the samples of rock A and rock B, which one is older? Justify your answer.
(b) The composition of the lead nucleus is unlikely to be greater than the uranium nucleus in the rocks sample. Explain your answer.
Answer:
(a)

A is the older sample. The ratio of uranium-238 to lead-206 is smaller.
(b)
The half-life of uranium-238 is 4.5 billion years. Therefore, the decay process of uranium -238 in a rock sample has gone through less than one half-life.
Hence, less than half of the uranium-238 nuclei in the sample of rock had decayed to form lead-206 nuclei.
So, the number of lead-206 nuclei cannot be more than the remaining uranium- 238 nuclei.
Question 5:
Carbon-14 has a half-life of 5 730 years.
(a) What is the fraction of undecayed carbon in a fossil sample at the end of 1.719 × 104 years?
(b) Based on your answer to 5(a), sketch a graph of the decay curve for carbon-14 in the fossil sample.
Answer:
(a)
$$ n=\frac{17190}{5730}=3 $$

(b)
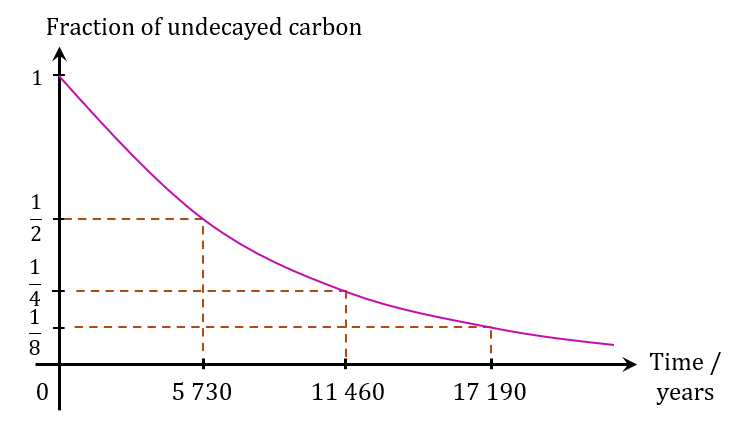
Carbon-14 has a half-life of 5 730 years.
(a) What is the fraction of undecayed carbon in a fossil sample at the end of 1.719 × 104 years?
(b) Based on your answer to 5(a), sketch a graph of the decay curve for carbon-14 in the fossil sample.
Answer:
(a)
$$ n=\frac{17190}{5730}=3 $$

After 1.719 × 104 years, 1/8 of carbon-14 in the sample has not decayed.
(b)

